STARR Site Certification
Over the last six years, a group of respected leaders from research sites, pharmaceutical companies, CROs, and advocacy have worked together to identify and develop resources and tools and have established some basic standards of practice for clinical research sites . Those sites that apply these tools and practices are eligible to be certified under the STARR Site Certification program.
The resources, tools, and standard practices have been compiled in the certification manual and listed here as individual modules.
The current modules offer tools and resources in the following areas: language, advocacy, community integration, suicide prevention, stigma reduction, and empathy training.
This certification is intended to be a living document. As new areas are identified, they will be work-grouped and implemented into the Certification. We welcome any input and suggestions on new areas, resources, and tools to help support and advance clinical research.
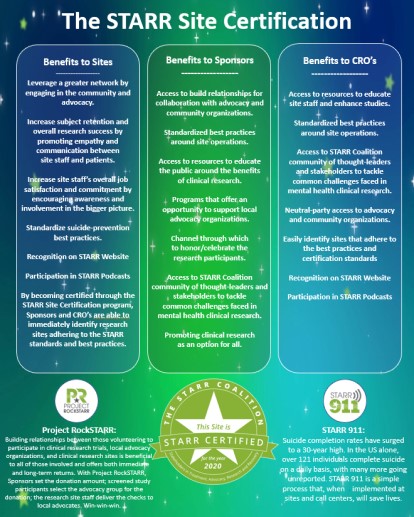
Why get STARR Certified?
- Join a community of sites focused on mental health research that work together to strengthen one another.
- Leverage a greater network by engaging in the community and advocacy.
- Increase subject retention and overall research success by promoting empathy and communication between site staff and patients.
- Increase site staff’s overall job satisfaction and commitment by encouraging awareness and involvement in the bigger picture.
- By becoming certified through this program, Sponsors and CRO’s are able to immediately identify research sites applying the tools and best practices established as part of the STARR Site Certification.
Still have questions? Check out the FAQs HERE.

Site Certification Pre-Assessment
The STARR Site Certification was created to promote empathy and communication between research site staff and patients, caregivers, and advocacy groups, as well as providing tools and support for best practices around areas identified by the stakeholders of The STARR Coalition.
The first step in the Site Certification
is the Pre-Assessment.
Click here to access the pre-assessment
Contact
The STARR Coalition is a resource for you. If you have any questions, concerns, or need assistance in any way, please reach out to us.
